Masimo
Evan Lamb
949-396-3376
elamb@masimo.com
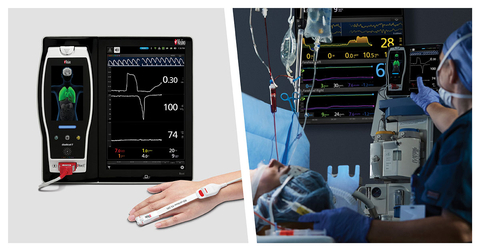
In the Largest Study of ORi to Date, of 554 Patients, Researchers Conclude: “ORi Can Provide Useful Information on Arterial Oxygenation Even During One-Lung Ventilation”
(BUSINESS WIRE) -- Masimo (NASDAQ: MASI) today announced the findings of a retrospective study published in the Journal of Anesthesia in which Dr. Yu Jeong Bang and colleagues at the Samsung Medical Center, Sungkyunkwan University School of Medicine, in Seoul, South Korea, investigated the association of Masimo ORi™ and the arterial partial pressure of oxygen (PaO2) in 554 patients who underwent non-cardiac thoracic surgery during one-lung ventilation (OLV), making this study the largest to date on ORi. The researchers found that ORi values “were significantly correlated with PaO2 measured simultaneously” and that ORi “could provide useful information on arterial oxygenation even during one-lung ventilation.”1
Noninvasive, continuous Masimo ORi provides continuous, real-time insight into the oxygenation of hemoglobin in the moderate hyperoxic range (PaO2 > 100 and ≤ 250 mmHg) to be used alongside arterial blood gas analyses, which have the drawbacks of being invasive, intermittent, and delayed. ORi is trended continuously with SpO2 as a unit-less index between 0.00 and 1.00 to extend the visibility of the patient oxygenation beyond SpO2 under supplemental oxygen. By convention, SpO2 is limited to an upper limit of 100%, but oxygenation is not limited and can rise into hyperoxia (higher than normal oxygenation state) when supplemental oxygen is administered. ORi provides clinicians with additional visibility, as a complement to SpO2 monitoring with Masimo SET® pulse oximetry, into when oxygenation is increased into, or decreased out of, moderate hyperoxia, in real time.
Noting the importance of striving to prevent hyperoxemia and hypoxemia especially during surgery requiring OLV, because of the risk of pulmonary complications, the researchers sought to evaluate a noninvasive, continuous method of predicting imminent over- or under-oxygenation to overcome the drawbacks of invasive blood gas analysis alone, using Masimo ORi. To evaluate ORi’s performance, they analyzed data collected from 554 patients who underwent non-cardiac elective thoracic surgery requiring OLV between January 1 and December 31, 2022 at a tertiary hospital in South Korea. During anesthesia, ORi was monitored using Masimo RD rainbow SET® Pulse CO-Oximetry sensors, and blood gas analysis was performed 15 minutes after OLV was initiated. The researchers’ primary endpoint was the association between simultaneous ORi and PaO2 values. They also sought to identify risk factors for PaO2 < 150 mmHg, based on their clinical experience that most patients with PaO2 > 150 mmHg in this scenario rarely show hypoxemia.
The researchers found a linear correlation between ORi and PaO2 measured simultaneously. Using linear regression analysis, they found there was a statistically significant positive relationship between ORi and PaO2 measured 15 minutes after OLV initiation (r2 = 0.5752, p < 0.001). Using receiver-operated curve (ROC) analysis, they identified an optimal cut-off ORi value of 0.27 to detect PaO2 ≥ 150 mmHg during OLV (area under the ROC curve of 0.96, 95% confidence interval of 0.94 – 0.98, sensitivity 0.909, specificity 0.932). Of the 11 potential predictors for PaO2 < 150 mmHg identified by the researchers, ORi was highly predictive (odds ratio 0.001, p < 0.001).
The researchers concluded, “ORi values during one-lung ventilation were significantly correlated with PaO2 measured simultaneously. Therefore, the ORi monitor can provide useful information for estimating the PaO2 value even during one-lung ventilation.”
In the U.S., ORi has been granted a De Novo by the FDA to be used in patients undergoing surgery as an adjunct to SpO2 for increased monitoring resolution of elevated hemoglobin oxygen saturation levels (e.g., due to administration of supplemental oxygen). The ORi feature is indicated for the monitoring of hemoglobin oxygen saturation levels in patients 18 years and older (adults and transitional adolescents) on supplemental oxygen during no-motion conditions perioperatively in hospital environments.
@Masimo | #Masimo
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-8 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,9 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2022-23 U.S. News and World Report Best Hospitals Honor Roll.10 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97® and Masimo W1®. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius Tº®, Masimo W1, and Masimo Stork™. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature/.
RPVi has not received FDA 510(k) clearance and is not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
Bang Y, Seong Y, Jeong H. Association between Oxygen Reserve index nd arterial partial pressure of oxygen during one-lung ventilation: a retrospective cohort study. J Anesth. 7 Sept 2023. DOI: 10.1007/s00540-023-03259-4
Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
Estimate: Masimo data on file.
http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo ORi™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo ORi, contribute to positive clinical outcomes and patient safety; risks that the researchers’ conclusions and findings may be inaccurate; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
View source version on businesswire.com: https://www.businesswire.com/news/home/20231210873994/en/
Masimo
Evan Lamb
949-396-3376
elamb@masimo.com