Masimo
Evan Lamb
949-396-3376
elamb@masimo.com
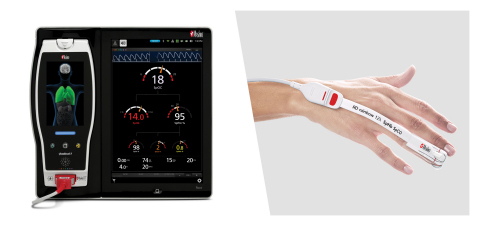
Masimo Launches Single-patient-use rainbow® SuperSensor™
- NEUCHATEL, Switzerland - Tuesday, 14. September 2021
- AETOSWire
One Patient, One Sensor, 12 Parameters: Breakthrough Technology Offers Advanced Physiological Insight in One Comprehensive, Convenient, Noninvasive Fingertip Solution
(BUSINESS WIRE)-- Masimo (NASDAQ: MASI) announced today the CE marking and commercial launch in Europe of the single-patient-use adhesive rainbow® SuperSensor™, compatible for use with both Masimo and third-party monitors with Masimo rainbow® technology inside. In an industry first, the comprehensive, convenient, and multi-purpose SuperSensor uses 12 LEDs to simultaneously offer 12 blood constituent parameters noninvasively and continuously: SET® oxygen saturation (SpO2), total hemoglobin, SpHb®, carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), oxygen reserve index (ORi™), PVi®, RPVi™, pulse rate (PR), respiration rate (RRp®), perfusion index (Pi), fractional oxygen saturation (SpfO2™), and oxygen content (SpOC™)—all on the same single-patient-use adhesive sensor. By allowing clinicians to noninvasively and continuously monitor so many different physiologic indicators simultaneously, the SuperSensor offers the ability to assess the patient’s status continuously.
At the core of the SuperSensor is Masimo SET® pulse oximetry, which has been clinically proven to help care teams enhance patient safety and improve patient outcomes; in fact, more than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies in clinical settings and motion and low perfusion conditions, providing clinicians with increased sensitivity and specificity to make critical care decisions.1 SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce deaths due to opioid overdose, while also reducing rapid response team activations, ICU transfers, and the cost of care.4-7
Continuous hemoglobin monitoring with SpHb as part of patient blood management programs has been found to help clinicians improve outcomes in both high- and low- blood loss surgeries, such as reducing the percentage of patients receiving transfusions,8 reducing the units of red blood cells transfused per patient,9-10 reducing the time to transfusion,11 and reducing costs.12 The utility of PVi, a measure of the dynamic changes in perfusion index that occur during the respiratory cycle, as an indicator of fluid responsiveness, has been demonstrated in more than 100 independent studies.13 Use of SpHb and PVi together, as part of an integrated goal-directed therapy protocol for fluid management and blood administration, has even been shown to help clinicians reduce mortality 30 and 90 days after surgery, by 33% and 29%, respectively.14
ORi is a noninvasive and continuous trending index that extends oxygen monitoring of patients receiving supplemental oxygen. By monitoring oxygenation beyond the upper limits of conventional pulse oximetry, ORi offers the potential for advanced warning of hypoxemia, during preoxygenation and intubation procedures, and of hyperoxia, in patients receiving greater concentrations of supplemental oxygen than clinically required. For example, in a study of pediatric patients undergoing general anaesthesia with orotracheal intubation, researchers found that ORi detected impending desaturation in a median of 31.5 seconds before noticeable changes in SpO2 occurred.15 A study evaluating the ability of ORi to predict mild hypoxemia during endotracheal intubation found that the time between decrease in ORi and subsequent decrease in SpO2 “may allow preventive action,” and that a higher ORi value during preoxygenation was “independently protective against hypoxemia.”16 In another study, researchers found that monitoring adult ICU patients with ORi significantly reduced the time these critically ill patients spent with moderate hyperoxia, compared to monitoring with oxygen saturation (SpO2) alone.17
SpMet helps clinicians noninvasively and continuously monitor methemoglobin levels in the blood.18 Elevated methemoglobin levels can be caused by many drugs given in hospitals, including inhaled nitric oxide (iNO) therapy,19-20 which has been used as a potential treatment for lung complications associated with COVID-19. SpMet may be an important monitoring tool during iNO therapy.
Dr. Max Jonas, Consultant in Intensive Care Medicine and Anesthesia at University Hospitals, Southampton, UK, commented, “Critically ill patients are frequently hemodynamically unstable, with variable oxygen delivery, which may be inadequate and lead to a cumulative oxygen debt, especially with noradrenaline infusions. Clinically this makes continuous monitoring and optimization of oxygen delivery using hemoglobin, fluid responsiveness, and oxygen saturation extremely important. It is also clinically invaluable being able to recognize impaired oxygen carriage, and hence content in the blood, for instance the methemoglobinemia generated by inhaled nitric oxide therapy, which we are currently frequently using during the treatment of COVID-19 pneumonitis and also for pulmonary hypertension.”
SpCO enables quick and noninvasive monitoring of carbon monoxide levels in the blood, and may lead to the identification of elevated CO levels that might otherwise go unnoticed in front-line settings such as fire rescue and mass casualty scenarios.21,22 Studies of emergency room patients have shown that SpCO may be a valuable tool for monitoring a large number of patients for possible CO exposure.23,24 For example, in a study of emergency room patients, of 32 patients diagnosed with CO poisoning, 22 would not have been identified without SpCO monitoring.25
By making SpMet, SpCO, and SpfO2 available on the same sensor, the SuperSensor provides a more complete picture of oxygenation in the presence of potential dyshemoglobin interference. Fractional oxygen saturation (FO2Hb) provides a measure of the fraction of total hemoglobin that is currently oxygenated, as opposed to SpO2, functional oxygen saturation, which measures the fraction of hemoglobin that is oxygenated based on an estimation of the effective hemoglobin available (hemoglobin capable of being oxygenated). In healthy individuals, FO2Hb is often similar to SpO2, but when dyshemoglobin levels are elevated, FO2Hb may be more representative of the total oxygen-carrying capacity of hemoglobin than SpO2. In the presence of dyshemoglobins, SpO2 may appear “normal,” but SpfO2—a noninvasive, continuous measurement of FO2Hb—may provide more insight into a possible oxygenation impairment. Combined with the ability to monitor SpCO and SpMet on the same sensor, clinicians now have additional information to help determine if a dyshemoglobin species is responsible, and intervene appropriately.
Dr. Anne Booth, Consultant in Neuroanaethesia and PHEM at Cambridge University Hospitals, and Joint Clinical Lead – Adult Critical Care Transfers, East of England, stated, “When assessing patients’ oxygenation, we need to be prepared for the unknown, especially in Emergency and Critical Care. If there are patients that have elevated carboxyhemoglobin from prior history of smoking or from carbon monoxide exposure, their oxygen content would be impaired. Similarly, with inhaled nitric oxide therapy, which is commonly used for COVID-19, patients may be subject to high methemoglobin levels. Parameters provided on the Masimo SuperSensor, like oxygen content (SpOC) and fractional oxygen saturation (SpfO2), can help us identify the source of diminished oxygen delivery so we can react accordingly.
Dr. Aryeh Shander, Anesthesiologist and an expert in patient blood management, commented, “A key focus in our care for critically ill patients during surgery and beyond is to minimize oxygen consumption and maximize oxygen utilization. The information now available noninvasively, via a pulse oximetry-like sensor, on total hemoglobin concentration, fluid responsiveness, dyshemoglobin presence, fractional oxygen saturation, oxygen content, and more, can give us much needed and critical information to help in providing the best clinical judgements. The ultimate goal is to improve the patient’s outcome and not just treat a number.”
Dr. Kiyoyuki Miyasaka, Anesthesiologist at the National Center for Child Health and Development, Tokyo, Japan, noted, “Pulse oximetry has progressed greatly since its invention in Japan in the 1970s. With the latest sensors from Masimo, clinicians now have greater visibility of the patient’s overall oxygen delivery, helping us better understand the underlying physiology impacting their condition. I look forward to seeing how Masimo pulse oximetry can further improve patient care.”
Joe Kiani, Founder and CEO of Masimo, said, “The SuperSensor represents a key milestone in Masimo’s continued innovation journey, giving clinicians access to 12 breakthrough noninvasive measurements in a single, convenient, and comprehensive sensor as part of our RD rainbow SET® sensor family—while easing concerns about cross-contamination because of being a single-patient-use, adhesive product. I am proud of our team for delivering this innovation to the medical community.”
SpHb and SpMet monitoring are not intended to replace laboratory blood testing. Blood samples should be analyzed by laboratory instruments prior to clinical decision making. Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgment considering among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples. SpCO monitoring is not intended to be used as the sole basis for making diagnosis or treatment decisions related to suspected carbon monoxide poisoning. It is intended to be used in conjunction with additional methods of assessing clinical signs and symptoms.
The accuracy of PVi in predicting fluid responsiveness is variable and influenced by numerous patient, procedure, and device-related factors. PVi measures the variation in the plethysmography amplitude but does not provide measurements of stroke volume or cardiac output. Fluid management decisions should be based on a complete assessment of the patient’s condition and should not be based solely on PVi. In the U.S., PVi is cleared as a noninvasive, dynamic indicator of fluid responsiveness in select populations of mechanically ventilated adult patients.
ORi, RPVi and SpfO2 have not received FDA 510(k) clearance and are not available for sale in the United States.
@Masimo | #Masimo
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,26 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.27 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient’s physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica™, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature/.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedic Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
- Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
- Imaizumi et al. Continuous and noninvasive hemoglobin monitoring may reduce excessive intraoperative RBC transfusion. Proceedings from the 16th World Congress of Anaesthesiologists, Hong Kong. Abstract #PR607.
- Kamal AM et al. The Value of Continuous Noninvasive Hemoglobin Monitoring in Intraoperative Blood Transfusion Practice During Abdominal Cancer Surgery. Open J Anesth. 2016;13-19.
- Ribed-Sánchez B et al. Economic Analysis of the Reduction of Blood Transfusions during Surgical Procedures While Continuous Hemoglobin Monitoring is Used. Sensors. 2018, 18, 1367; doi:10.3390/s18051367.
- Published clinical studies on PVi, with varying results and outcomes, can be found on our website at http://www.masimo.com/evidence/pulse-oximetry/pvi. Studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Cros J et al. Continuous hemoglobin and plethysmography variability index monitoring can modify blood transfusion practice and is associated with lower mortality. J Clin Monit Comp. 3 Aug 2019. https://doi.org/10.1007/s10877-019-00367-z.
- Szmuk P et al. Anesthesiology. 2016; 124:00-00.
- Hille H, Le Thuaut A, Canet E, Lemarie J, Crosby L, Ottavy G, Garret C, Martin M, Seguin A, Lamouche-Wilquin P Morin J, Zambon O, Miaihle AF, Reignier J, Lascarrou JB. Oxygen reserve index for noninvasive early hypoxemia detection during endotracheal intubation in intensive care: the prospective observational NESOI study. Ann. Intensive Care. 2021 11:112. DOI: 10.1186/s13613-021-00903-8.
- Lasocki S, Brochant A, Leger M, Gaillard T, Lemarié P, Gergaud S, and Dupré P. ORi monitoring allows a reduction of time with hyperoxia in critically ill patients: the randomized control ORi study. Intensive Care Med. 13 Aug 2019. https://doi.org/10.1007/s00134-019-05732-9.
- Annabi E et al. Severe Methemoglobinemia Detected by Pulse Oximetry. Anesth Analg. 2009 Mar;108(3):898-9.
- Riou Y et al. Pediatric Research. 1998. 43, 295-295.
- U.S. Food & Drug, Consumer Updates, Benzocaine and Babies: Not a Good Mix.
- Augustine JJ. JEMS. 2007 May;64-71.
- Bledsoe BE et al. Prehosp Emerg Care. 2010 Jan-Mar;14(1):131-3.
- Suner S et al. J Emerg Med. 2008 May;34(4):441-50.
- Roth D et al. Ann Emerg Med. 2011 Jul;58(1):74-9.
- Roth D et al. Int J Clin Pract. 2014 Oct;68(10)1239.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo rainbow® SuperSensor™ and its utility of its 12 provided parameters. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo rainbow® SuperSensor, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210912005064/en/
