Masimo
Evan Lamb
949-396-3376
elamb@masimo.com
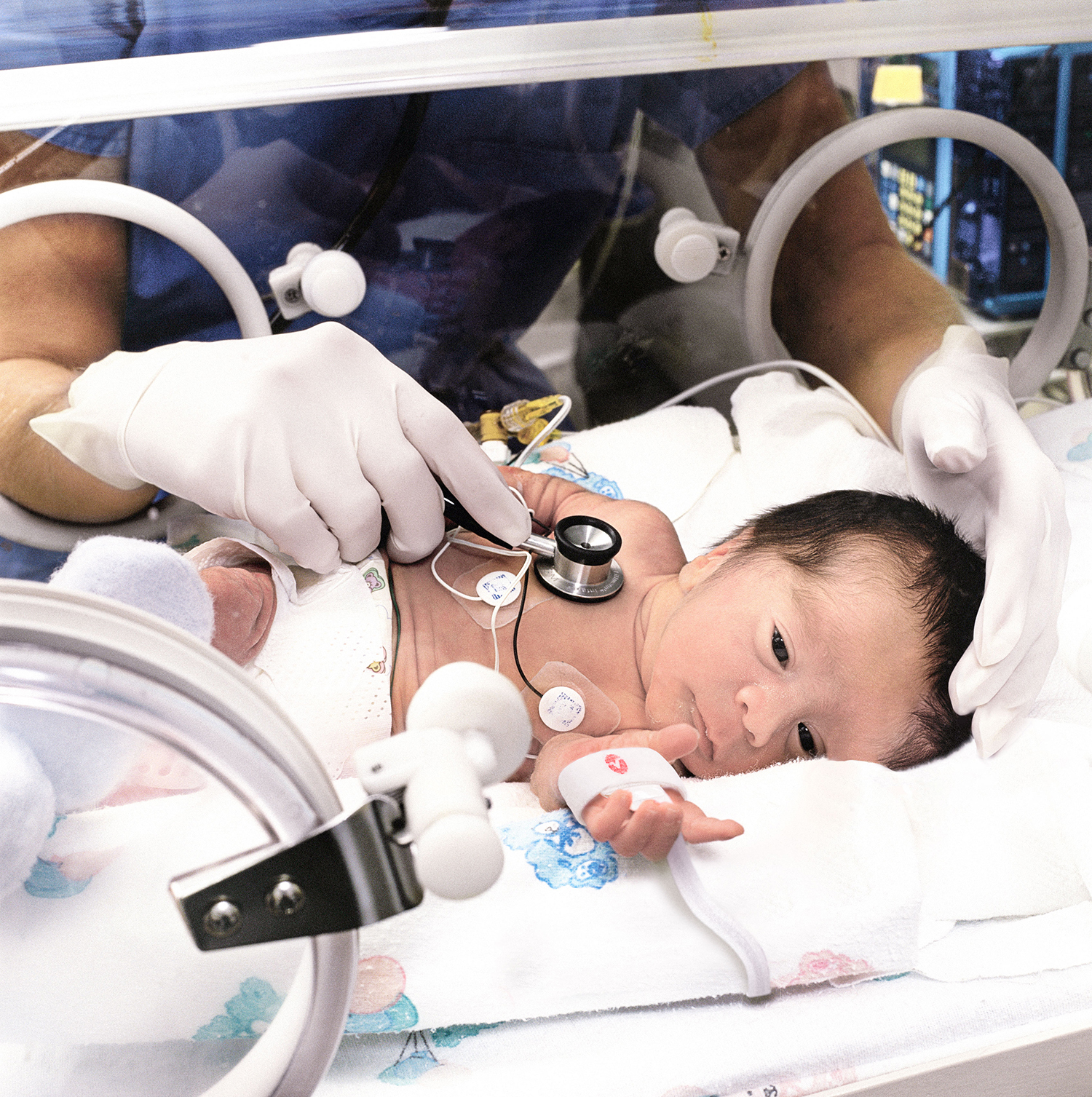
Masimo Monitoring Solutions Promote Newborn and Maternal Safety
- NEUCHATEL, Switzerland - Thursday, 11. March 2021
- AETOSWire
New Neonatal Study Adds to Body of Clinical Evidence Demonstrating Masimo SET® Pulse Oximetry’s Unique Ability to Improve Care
(BUSINESS WIRE)-- Masimo (NASDAQ: MASI) provides a variety of innovative monitoring solutions designed to improve maternal and newborn safety during childbirth and the critical first minutes of life. Masimo SET® pulse oximetry’s ability to measure during motion and low perfusion has helped newborns, neonates, and pediatric patients like no other pulse oximetry. Not only has Masimo SET® helped clinicians reduce retinopathy of prematurity (ROP)1 and improve screening for critical congenital heart disease (CCHD),2-10 but it has helped push the standard of care for babies to new heights—the evidence from CCHD studies with SET®, for example, has been used in the establishment of screening guidelines used around the world.11
Today, on International Women’s Day, it is especially important to recognize that, as UNICEF reports, “newborns and mothers are still dying in appalling numbers.” Every day, approximately 7,000 babies in the first month of life die, and approximately 810 women die, from preventable complications related to childbirth or pregnancy.12 Similarly, according to the WHO, “Although important progress has been made in the last two decades, about 295,000 women died during and following pregnancy and childbirth in 2017. The most common direct causes of maternal injury and death are excessive blood loss, infection, high blood pressure, unsafe abortion, and obstructed labor, as well as indirect causes such as anemia, malaria, and heart disease.”13
Masimo’s first and ongoing focus has been helping neonatologists, pediatricians, OB-GYNs, and midwives around the world provide the best care possible for newborns and their mothers. The Masimo Newborn Sensor, the first and still the only sensor of its kind, was introduced in 2004 and is designed to provide accurate arterial oxygen saturation (SpO2) and pulse rate (PR) measurements in the fastest time possible during hectic neonatal resuscitation scenarios. Alongside Newborn Sensors, Pathway™, introduced in 2019 for the Root® platform, helps clinicians visualize their preferred SpO2 and PR protocol during neonatal resuscitation. Eve™, a software application introduced in 2014, simplifies and automates the CCHD screening process, which Masimo SET® enabled. The Blue® Sensor, introduced in 2004, provides accurate monitoring in cyanotic children at low SpO2 levels to help clinicians care for them and was validated specifically on infants with cyanotic disease.14 rainbow® SpHb®, noninvasive hemoglobin monitoring, introduced in 2008, can measure hemoglobin levels during pregnancy and alert clinicians to the possibility of excessive blood loss during delivery.
Adding to the significant body of clinical evidence demonstrating the utility of SET® pulse oximetry and other Masimo newborn and maternal solutions, a new study published in the Journal of Clinical Neonatology investigated the use of comparing Masimo perfusion index (Pi) pre- and post-ductal values on pre-term infants to aid clinicians in diagnosing hemodynamically significant patent ductus arteriosus (hsPDA).15 Dr. Melek Büyükeren and colleagues at Hacettepe University in Ankara, Turkey found that the difference in right-hand and right-leg Pi values obtained using Masimo SET® pulse oximetry was significantly higher in pre-term infants with hsPDA, leading them to conclude that the difference in Pi “has diagnostic value in hsPDA and can assist diagnosis when echocardiography is not available.”
Marcelo Cardetti, MD, said, “As Head of the Neonatology Service of the Clinic and Maternity of the Center for Endocrinology and Human Reproduction (CERHU) in San Luis, Argentina, we have been using Masimo pulse oximetry monitors with SET® for approximately 8 years in all high-risk newborns and also for newborn resuscitation. In addition, we use Masimo SET® monitors for the detection of CHD and hypoxemia in all newborns in the mother-infant unit. Furthermore, our neonatal department is engaged in a research protocol on regional cerebral oxygenation (O3®) with neonatal sensors for Masimo Root - to know what happens with cerebral oxygenation during routine clinical procedures in the NICU. This monitoring, in addition to SpO2 and perfusion index (Pi), perfectly shows us what is happening with oxygenation of seriously ill newborns in real-time and in a noninvasive way. Masimo SET®’s innovative technology far overcomes the limitations of conventional oximetry and the Pi is an important clinical tool in the care of sick neonates. This monitor and the special neonatal RD sensors have been of great value for the prevention of ROP and for successful and quick, accurate, and reliable steps needed in resuscitation in the delivery room.”
Hernando Baquero, MD, commented, “I am a pediatrician, neonatologist, clinician, educator and researcher in a major university in Colombia, with several publications on noninvasive neonatal SpO2 monitoring and oxygenation. The introduction in the Latin American market of Masimo SET® technology dramatically improved neonatal care in our countries. In contexts with serious resource limitations, as is the case in most neonatal units in our countries, it was vital to be able to provide quality care to the most vulnerable population due to their health conditions (e.g. hypoperfusion) or their biological characteristics (e.g. prematurity). Having reliable, fast, and stable readings as provided by SET® and its neonatal sensors improved the chances of many of our newborns.”
Anne de-Wahl Granelli, PhD, Biomedical Scientist, RDCS(PE), Medical Centre Manager, Sweden, said, “The integration of pulse oximetry into the CCHD screening process has made a significant impact on the detection of congenital heart disease and neonatal health. In clinical studies, the use of pulse oximetry screening with SET® technology significantly improved the detection of duct dependent heart disease before hospital discharge. In 2011, the U.S. Department of Health and Human Services added pulse oximetry screening of newborns for CCHD to the Recommended Uniform Screening Panel. Today pulse oximetry has become a global standard of care when screening newborns for CHD.”
Sergio Golombek, MD, MPH, FAAP, Member of the Board and Past President of the Ibero-American Society of Neonatology (SIBEN), said, “I have authored and published several scientific studies in relation to newborn oxygenation and screening for CCHD. Masimo SET® and new innovations and sensor development like RD sensors for noninvasive monitoring represent excellent technology that we can trust, that work promptly and accurately when we need it the most, and are designed specifically with ill newborn babies in the NICU in mind. SET® technology allows us also to do the pulse oximetry test or CCHD screening on newborn babies in our units, knowing well that we can fully trust the results. The technology is very easy to use and understand, and makes us deliver better clinical care.”
Katsuyuki Miyasaka, MD, PhD, Executive Advisor, Wayo Women’s University Graduate School, Tokyo and Professor Emeritus, St. Luke’s International University, Tokyo, said, “As a critical care pediatric anesthesiologist, reliable and accurate pulse oximetry is paramount to optimal patient outcomes. Some suggest pulse oximetry is the fifth vital sign. Clinicians can rely on the sensitivity and specificity provided by Masimo’s measure-through-motion technology in the management of children in the PICU. The use of pulse oximetry can lead to fewer adverse events in the recovery room by capturing accurate readings even during movement such as shivering in critically ill or unstable patients.”
Mark Ansermino, MBBCh, MMed, Director of the Center for International Child Health and Professor, Department of Anesthesiology, Pharmacology & Therapeutics at the University of British Columbia, Canada, said, “Anemia is a significant public health problem that especially affects the quality of life, health status, and survival of mothers and children around the world. Having access to continuous hemoglobin monitoring technology can help provide visibility to hemoglobin levels. The noninvasive nature of the SpHb solution makes it comfortable for the mother and child and makes monitoring during childbirth feasible even in low-resource settings.”
Asrat Dibaba Tolossa, MD, MPH, is Chief of Party for the Global Affairs Canada ENRICH (Enhancing Nutrition Services to Improve Maternal and Child Health) Program, a multi-year, multi-country initiative designed to improve the health and nutrition of mothers, newborns, and children. As part of the program, ENRICH has been conducting a study in central Tanzania, where maternal and child care services are often overburdened, using the Masimo Rad-67® Pulse CO-Oximeter®, which provides spot-check SpHb measurements. Dr. Tolossa commented, “In our field experimentation with the Rad-67, we found out that the device can be used easily by lower-level health workers in the communities for screening and referral of patients to health facilities for further assessment and treatment. There was also a high acceptance rate by community members as the method is noninvasive.”
Joe Kiani, Founder and CEO of Masimo, said, “From our inception, we have been committed to improving outcomes for the youngest and most fragile patients. Our foundational SET® pulse oximetry was designed with newborns in mind. With rainbow® Pulse CO-Oximetry, we have made the noninvasive monitoring of child and mother clinically more meaningful. While we stand behind the fact that we have the best pulse oximetry for all patients, especially the most fragile patients, we continue to seek new ways to help clinicians provide newborns and their mothers with the best care possible. On this International Women’s Day, we thank the caregivers who have dedicated themselves to the health of newborns and their mothers, as well as women everywhere, for their achievements, their sacrifices, and for nurturing us all.”
SpHb is not intended to replace laboratory blood testing. Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgment considering, among other factors, patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
Noninvasive, continuous SpHb has CE clearance for all patients and in the U.S. has received FDA clearance for patients >3 kg but is not currently indicated for patients <3 kg. As part of its U.S. FDA 510(k) clearance, spot-check SpHb on Rad-67 is contraindicated for use on pregnant patients and not indicated for use on pediatric patients or patients with renal disease. Eve has not obtained FDA clearance and is not available in the United States.
@Masimo | #Masimo
*ARMS accuracy is a statistical calculation of the difference between device measurements and reference measurements. Approximately two-thirds of the device measurements fell within +/- ARMS of the reference measurements in a controlled study.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.16 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.17-20 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,21 and is the primary pulse oximetry at 9 of the top 10 hospitals according to the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.22 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient’s physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature/.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- Slitine N, et al. Pulse Oximetry and Congenital Heart Disease Screening: Results of the First Pilot Study in Morocco. Int J Neonatal Screen 6(53). 30 June 2020.
- Zhao et al. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: a prospective study. Lancet. 2014 Aug 30;384(9945):747-54.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Ewer AK et al. Pulse Oximetry Screening for Congenital Heart Defects in Newborn Infants (Pulseox): A Test Accuracy Study. Lancet. 2011 Aug 27;378(9793):785-94.
- de-Wahl Granelli A et al. Noninvasive Peripheral Perfusion Index as a Possible Tool for Screening for Critical Left Heart Obstruction. Acta Paediatr 2007; 96(10): 1455-9.
- Meberg A et al. First Day of Life Pulse Oximetry Screening to Detect Congenital Heart Defects. J Pediatr 2008; 152:761-5.
- Schena F et al. Perfusion Index and Pulse Oximetry Screening for Congenital Heart Defects. J Pediatr. 2017 Apr;183:74-79.
- Hamilçıkan S, Can E. Critical Congenital Heart Disease Screening With a Pulse Oximetry in Neonates. J Perinat Med. 2018 Feb 23;46(2):203-207.
- Jawin V et al. Beyond Critical Congenital Heart Disease: Newborn Screening Using Pulse Oximetry for Neonatal Sepsis and Respiratory Diseases in a Middle-Income Country. PLoS One. 2015; 10(9): e0137580.
- Kemper et al. Strategies for implementing screening for critical congenital heart disease. Pediatrics. 2011 Nov;128(5):e1259-67. doi: 10.1542/peds.2011-1317.
- https://www.unicef.org/health/maternal-and-newborn-health
- https://www.who.int/health-topics/maternal-health#tab=tab_1
- Harris B et al. Ped Crit Care Med. 2016 Apr;17(4):315-20.
- Büyükeren M, Yiğit S, Aykan HH, Karagöz T, Çelik HT, Yurdakök M. Comparison of perfusion index and echocardiographic parameters in preterm infants with hemodynamically significant patent ductus arteriosus. J Clin Neonatol 2021;10:11-8.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of SET®, Newborn Sensors, Pathway™, Eve™, Blue®, rainbow®, and SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including SET®, Newborn Sensors, Pathway, Eve, Blue, rainbow®, and SpHb, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210307005058/en/
https://aetoswire.com/en/news/masimo-monitoring-solutions-promote-newborn-and-maternal-safety
